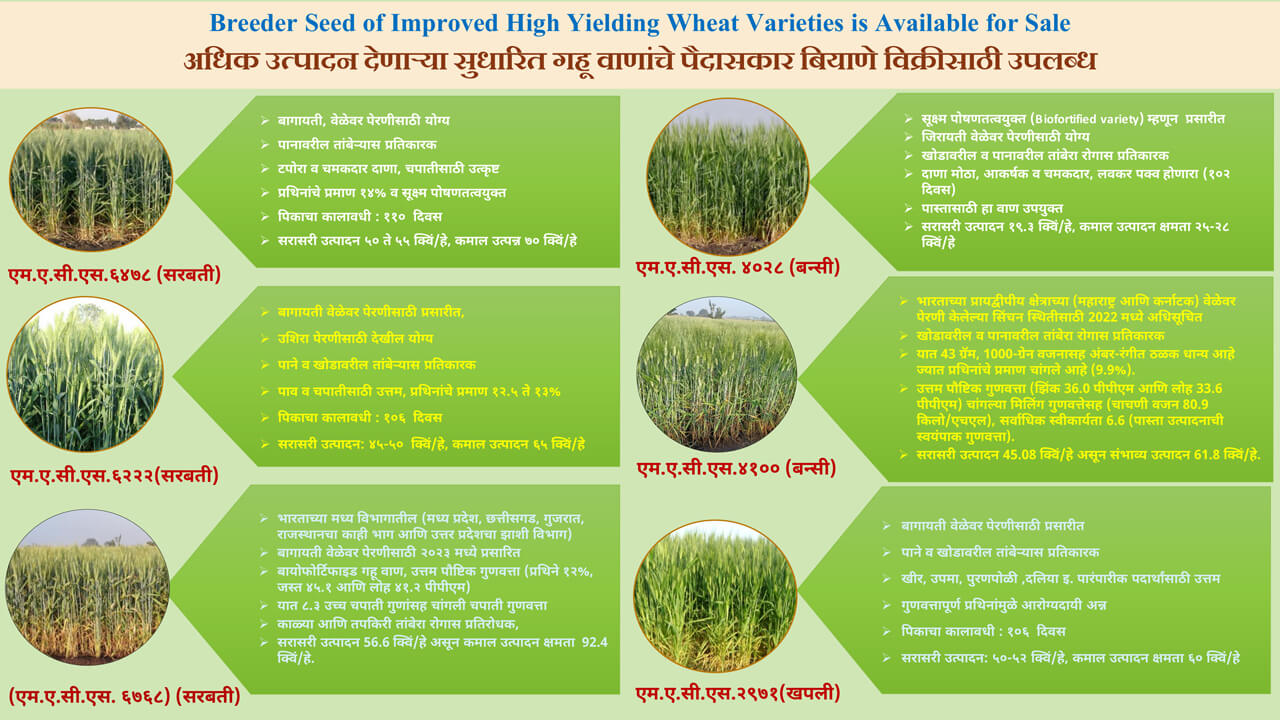
Google Scholar / Social Media
Name : Dr. Prasad Padmkar Kulkarni
Designation : Scientist – F
Brief Background :
Brief Background:Dr Prasad Kulkarni is working at Agharkar Research Institute, Pune, as Scientist F. DrKulkarni completed PhD in Chemistry from the Department of Chemistry, Savitribai PhulePune University. He has more than 20 years of research experience in Chemistry and Biochemistry. He did postdoctoral work at the Hospital for Sick Children, Canada, andRowett Research Institute, U.K. He was a British Council Visiting Fellow at the Departmentof Chemistry at the University of Hull. Currently, he is working at Bioprospecting Group,Agharkar Research Institute, Pune, India. He is a member of the Editorial Advisory Board ofRSC “Metallomics.” He is also a Life member of the Chemical Research Society of India.He is working on the role of copper in neurological disorders such as Menkes disease andAlzheimer’s disease. He is also working on iron-deficiency anemia and inflammation associated with anemia. He has over 50 publications and two patents. Recently, he hasbeen working on the sustainable utilization of medicinal plant resources in Maharashtra.
He has expertise in synthetic techniques: peptide synthesis, organic synthesis, andsynthesis of metal complexes of organic and peptide ligands. He has extensively used UV-visible, NMR, EPR, fluorescence, and CD spectroscopies and chromatographic techniquessuch as HPLC, GC-MS, and LC-MS. He also employs a variety of bio-analytical methodssuch as electrophoresis techniques, column chromatography, affinity chromatography,western blotting, confocal microscopy, and biological mass spectrometry (MALDI, ESI,SELDI) for protein identification in his research. He works with human and bacterial cellcultures and handles animal and human tissue samples.
Contact Details :
02025325102
Ph. D.(Chemistry), University of Pune; M. Sc.(Inorganic) Department of Chemistry, University of Pune, B. Sc., Modern College of Arts Commerce and Science, University of Pune, (Chemistry, Botany, Zoology)
More than 15 years of research experience in Bioinorganic chemistry and analytical biochemistry, specializing in chemistry and biochemistry of copper metabolism and copper-related metabolic disorders, metal ions, and oxidative stress in Alzheimer’s disease, iron deficiency anemia, and anemia of inflammation. My team is working on understanding therole of transition metals, especially Cu, Fe, Zn, and Pt, in neurodegenerative diseases suchas Alzheimer’s disease (AD).We are also working on the sustainable utilization of medicinal plant resources in Maharashtra. Maharashtra’s medicinal plant resources have immense potential torevolutionize healthcare and agriculture. We proposed to bridge the gap between traditional knowledge and sustainable practices. This work brings together stakeholders to standardize practices, identify bioactive principles, and design effective formulations.
- Separation of serum by paper based micro-fluidics and estimation of different forms of ironusing camera phone. P. P. Kulkarni, D. S. Bodas, B. N. Joshi. Indian Patent No. 413193(Application number 416/MUM/2014)
- Pyridinium oxazole dyad scaffold and a process for preparation thereof. N. T. Patil, A. C.Shaikh, P. P. Kulkarni, D. Ranade. U.S. Patent number 11021490 (Application number16802335)
Publications:
52. Badekar, P; Deo, H; Varma, M; Kulkarni, PP; Maibam, A; Krishnamurty, S; Kumbhar, A.(2024) ‘Turning On’to Glutathione: A Rhodamine‐Based Fluorescent Chemodosimeter withNanomolar Sensitivity. Chemistry Select, 9, e202402943.
51. Shete, P; Ghatpande, N; Varma, M; Joshi, P; Suryavanshi, K; Misar, A; Jadhav, S; Apte,P; Kulkarni, PP. (2024) Chronic dietary iron overload affects hepatic iron metabolism andcognitive behaviour in Wistar rats. Journal of Trace Elements in Medicine and Biology, 8,127422.
50. Sharon, N; Ugale, V; Padmaja, P; Lokwani, D; Salunkhe, C; Shete, P; Reddy, PN,Kulkarni, PP. (2024) Development of novel 9H-carbazole-4H-chromene hybrids as dualcholinesterase inhibitors for the treatment of Alzheimer’s disease. Molecular Diversity, 1-18
49. Ugale, V; Deshmukh, R; Lokwani, D; Reddy N P; Khadse, S; Chaudhari, P; Kulkarni, PP; (2024) GluN2B subunit selective N-methyl-D-aspartate receptor ligands: Democratizingrecent progress to assist the development of novel neurotherapeutics. Molecular Diversity,1765-1792.
48. Varma, M; Ugale, V; Shaukat, J; Hollmann, M; Shete, P; Shravage, B; Tayade, S;Kumbhar, A; Butcher, R; Jani, V; Sonavane, U; Joshi, R; Lokwani, D; Kulkarni, PP. (2023)Novel alkyl-substituted 4-methoxy benzaldehyde thiosemicarbazones: Multi-target directedligands for the treatment of Alzheimer’s disease. European Journal of Pharmacology, 957,176028.
47. Singh, S., Kumbhar, A, Walke G. & Kulkarni, P.P. (2022). An insight into the morphologyof DNA compaction induced by homobinuclear Ru (II) polypyridyl complexes. Journal ofInorganic Biochemistry, 234, 111870-111877.
46. Singh, S., Varma, M., Shravage, B., Kulkarni, P.P., & Kumbhar, A. (2021).Photoactivated cytotoxicity induced by heterobimetallic Ru (II)-Pt (II) polypyridyl complexesin MCF-7 cells. Journal of Chemical Sciences, 133(3), 1-12.
45. Kirtani, D. U., Ghatpande, N. S., Suryavanshi, K. R., Kulkarni, P. P., & Kumbhar, A. A. (2021). Fluorescent Copper (II) Complexes of Asymmetric Bis (Thiosemicarbazone) s:Electrochemistry, Cellular Uptake and Antiproliferative Activity. ChemistrySelect, 6(24),6063-6070.
44. Varma, M., Shravage, B., Tayade, S., Kumbhar, A., Butcher, R., Jani, V. and Kulkarni, P.P. (2021). A simple methyl substitution of 3-acetylcoumarin thiosemicarbazone enhancescellular autophagy flux, reduces inflammation and ameliorates rough eye phenotype in theDrosophila model of Alzheimer’ s disease. Journal of Molecular Structure, 1235, 130265.
43. Badekar, P. S., Thakur, G. C., Varma, M. E., Ghatpande, N. S., Kulkarni, P. P., A Kumbhar, A. A. (2021). Rhodamine‐Based Fluorescence’ Turn–on;Chemosensor: Detectionof Fe3+ Ion in Aqueous Medium and MCF‐7 Live Cells. ChemistrySelect, 6(9), 2373-2378.
42. Ghatpande, N. S., Misar, A. V., Waghole, R. J., Jadhav, S. H., Kulkarni, P. P. (2019).42. N. S. Ghatpande, A. V. Misar, R. J. Waghole, S. H. Jadhav, P. P. Kulkarni.
Tinosporacordifolia protects against inflammation associated anemia by modulating
inflammatorycytokines and hepcidin expression in male Wistar rats. Scientific reports 9 (1), 1-11, 2019
41. D. S. Yarramala, P. Prakash, D. S. Ranade, S. Doshi, P. P. Kulkarni, P. Bhaumik, C. P.Rao. Cytotoxicity of apo bovine α-lactalbumin complexed with La3+ on cancer cellssupported by its high-resolution crystal structure. Scientific Reports, 9, 1780-1791, 2019.
40. A. C. Shaikh, M. E. Verma, R. D. Mule, S. Banerjee, P. P. Kulkarni, N. T. Patil. IonicPyridinium-Oxazole Dyads: Design, Synthesis, and their Application in MitochondrialImaging. Journal of Organic Chemistry, 84, 1766–1777, 2019.
39. N. S. Ghatpande, P. P. Apte, S. S. Naik, P. P. Kulkarni. Fruits and vegetablesconsumption and their association with the indicators of iron and inflammation status amongadolescent girls. Journal of the American College of Nutrition, 38(3) 218-226, 2019.
38. A. Santoro, G. Walke, B. Vileno, P. P. Kulkarni, L. Raibaut, P. Faller. Low catalytic activityof the Cu (ii)-binding motif (Xxx-Zzz-His; ATCUN) in reactive oxygen species production andinhibition by the Cu (i)-chelator BCS. Chemical Communications, 54, 11945-11948, 2018.
37. N. A. Vyas, S. B. Singh, A. S. Kumbhar, D. S. Ranade, G. R. Walke, P. P. Kulkarni, V.Jani, U. B. Sonavane, R. R. Joshi, and S. Rapole. Acetylcholinesterase and Aβ AggregationInhibition by Heterometallic Ruthenium (II)–Platinum (II) Polypyridyl Complexes. InorganicChemistry, 57, 7524–7535, 2018.
36. J. Haribabu, D.S. Ranade, N.S.P. Bhuvanesh, P.P. Kulkarni, R. Karvembu. (2017) Ru (II)-p-cymene Thiosemicarbazone Complexes as Inhibitors of Amyloid β (Aβ) Peptide Aggregationand Aβ‐Induced Cytotoxicity. ChemistrySelect, 2, 11638-11644.
35. D.S. Ranade, B.V. Shravage, A.A. Kumbhar, U.B. Sonawane, V.P. Jani, R. Joshi, P. P.Kulkarni. Thiosemicarbazone Moiety Assist in Interaction of Planar Aromatic Moleculeswith Amyloid Beta Peptide and Acetylcholinesterase. ChemistrySelect, 2, 3911-3916, 2017.34.
34. G.R. Walke, D.S. Ranade, S.N. Ramteke, S. Rapole, C. Satriano, E. Rizzarelli, G.Tomaselli, G. T. Sfrazzetto, P. P. Kulkarni.(2017) Fluorescent Copper Probe Inhibiting Aβ1–16-Copper (II)-Catalyzed Intracellular Reactive Oxygen Species Production. InorganicChemistry 56, 3729-3732.
33. K. Samanta, D. S. Ranade, A. Upadhyay, P. P. Kulkarni, and C. P. Rao A Bimodal,Cationic, and Water-Soluble Calix[4]arene Conjugate: Design, Synthesis, Characterization,and Transfection of Red Fluorescent Protein Encoded Plasmid in Cancer Cells. ACS AppliedMaterials; Interfaces, 9, 5109–5117, 2017.
32. A. C. Shaikh, Ds. S. Ranade, P. R. Rajamohanan, P. P. Kulkarni, and N. T. Patil.(2016) Oxidative Intramolecular 1,2-Amino-oxygenation of Alkynes under Au(I)/Au (III)-Catalysis:Discovery of Pyridinium-Oxazole Dyadas Novel Ionic Fluorophore. AngewandteChemie,129, 775-779.
31. N. S. Ghatpande, P. P. Apte, B. N. Joshi, S. S. Naik, D. S. Bodas, V. Sande, P.Uttarwar, P. P. Kulkarni. Development of a novel smartphone-based application foraccurate and sensitive on-field hemoglobin measurement. RSC Advances, 6, 104067
30. N. A Vyas, S. N. Ramteke, A. S. Kumbhar, P. P. Kulkarni, V. Jani, U. B. Sonawane, R. R.Joshi, B. Joshi, A. Erxleben. (2016) Ruthenium (II) polypyridyl complexes with hydrophobic ancillaryligand as Aβ aggregation inhibitors. European Journal of Medicinal Chemistry, 121, 793-802
29. G. R. Walke, D. S. Ranade, A. M. Bapat, R. Srikanth, P. P. Kulkarni. (2016). Mn (III)‐SalenProtect Against Different ROS Species Generated by the Aβ16‐Cu Complex.ChemistrySelect, 1, 3497-3501,
28. M. R. Hardikar, M. E. Varma, A. A. Kulkarni, P. P. Kulkarni, B. N. Joshi. (2016) Elucidation ofhypoglycemic action and toxicity studies of insulin-like protein from Costusigneus. Phytochemistry, 124, 99-107.
27. Y. P. Ginotra, S. N. Ramteke, G.R Walke, S. Rapole, P. P. Kulkarni. (2016) Histidine availabilityis decisive in ROS-mediated cytotoxicity of copper complexes of Aβ1–16 peptide. Freeradical research, 50, 405-413.
26. L. B. Rane, A. N. Kate, S. N. Ramteke, B. V. Shravage, P. P. Kulkarni, A. A. Kumbhar. (2016) Fluorescent zinc (ii) complexes for gene delivery and simultaneous monitoring of proteinexpression. Dalton Transactions, 45, 16984-16996.
25. B. Shravage, S. Ramteke, P. P. Kulkarni, D. S. Bodas. (2016) A concave microwell arrayfabricated using the ommatidium of the common fruit fly for efficient cell culture. RSCAdvances, 6, 64266-64270.
24. N. S. Ghatpande, P. P. Apte, S. S. Naik, B. N. Joshi, M. K. Gokhale, P. P. Kulkarni. (2016) Association of B 12 deficiency and anemia synergistically increases the risk of high TNF-αlevels among adolescent girls. Metallomics, 8, 734-738.
23. D. S. Ranade, A. M. Bapat, S. N. Ramteke, B. N. Joshi, P. Roussel, A. Tomas, P.Deschamps, P. P. Kulkarni. (2015) Thiosemicarbazone modification of 3-acetyl coumarin inhibitsAβ peptide aggregation and protect against Aβ-induced cytotoxicity. European journal ofmedicinal chemistry, 121, 803-809.
22. A. C. Shaikh, D. S. Ranade, S. Thorat, A. Maity, P. P. Kulkarni, R. G. Gonnade, P.Munshi, N. T. Patil. (2015) Highly emissive organic solids with remarkably broad colortunabilitybased on N, C-chelate, four-coordinate organoborons. Chemical Communications, 51,16115-16118.
21. S. N. Ramteke, G. R. Walke, B. N. Joshi, S. Rapole, P. P. Kulkarni. (2014) Effects of oxidationon redox and cytotoxic properties of copper complex of Aβ1–16 peptide. Free radicalresearch, 48, 1417-1425.
20. G. R. Walke, S. Rapole, P. P. Kulkarni. (2014) Cisplatin inhibits the formation of a reactiveintermediate during copper-catalyzed oxidation of amyloid β peptide. Inorganic chemistry, 53,10003-10005.
19. N. A. Vyas, S. S. Bhat, A. S. Kumbhar, U. B. Sonawane, V. Jani, R. R. Joshi, S. N.Ramteke, P. P. Kulkarni, B. Joshi. (2014) Ruthenium (II) polypyridyl complex as inhibitor ofacetylcholinesterase and Aβ aggregation. European journal of medicinal chemistry, 75, 375- 381.
18. G. R. Dhage, S. R. Thopate, S. N. Ramteke, P. P. Kulkarni. (2014) One-pot synthesis andevaluation of novel 3-aryl-6-ethoxycarbonyl-4-hydroxy-2 H-pyran-2-one as a potent cytotoxicagent. RSC Advances, 4, 56870-56875.
17. S. N. Ramteke, Y. P. Ginotra, G. R. Walke, B. N. Joshi, A. S. Kumbhar, S. Rapole, P. P.Kulkarni. (2013) Effects of oxidation on copper-binding properties of Aβ1-16 peptide: A pulseradiolysis study. Free radical research, 47, 1046-1053.
16. Y. P. Ginotra, S. N. Ramteke, R. Srikanth, P. P. Kulkarni. (2012) Mass Spectral Studies Revealthe Structure of Aβ1–16–Cu2+ Complex Resembling ATCUN Motif. Inorganic chemistry, 51; 7960-7962.
15. Y. P. Ginotra, P. P. Kulkarni. (2007) Solution structure of physiological Cu (His) 2: novelconsiderations into imidazole coordination. Inorganic chemistry, 48, 7000-7002.
14. S. Narindrasorasak, P. P. Kulkarni, P. Deschamps, Y. She, B. Sarkar. (2007) Characterizationand copper binding properties of human COMMD1 (MURR1). Biochemistry, 46, 3116-3128.
13. P. P. Kulkarni, Y. She, S. D. Smith, E. A. Roberts, B. Sarkar. (2006) Proteomics of MetalTransport and Metal‐Associated Diseases. Chemistry–A European Journal, 12, 2410-2422.
12. P. Deschamps, P. P. Kulkarni, M. Gautam-Basak, B. Sarkar. (2005) The saga of Copper (II)–l-histidine. Coordination chemistry reviews, 249, 895-909.
11. Z. Afrasiabi, E. Sinn, P. P. Kulkarni, V. Ambike, S. Padhye, D. Deobagakar, M. Heron, C.Gabbutt, C. E. Anson, A. K. Powell. (2005) Synthesis and characterization of Copper (II) complexesof 4-alkyl/aryl-1, 2-naphthoquinones thiosemicarbazones derivatives as potent DNA cleavingagents. Inorganicachimica acta, 358, 2023-2030.
10. P. Deschamps, P. P. Kulkarni, B. Sarkar. (2003) X-ray structure of physiological copper (II)-bis(L-histidinato) complex. Inorganic chemistry, 43, 3338-3340.
9. P. Deschamps, P. P. Kulkarni, B. Sarkar. (2003) The crystal structure of a novel copper (II)complex with asymmetric ligand derived from l-histidine. Inorganic chemistry, 42, 7366-7368.
8. P. P. Kulkarni, S. Padhye, E. Sinn. Hemiprotonateddafone, a new cationic species. (2003) The novel di-dafonium iron (III) complex: [(dafone) 2 H] + [FeCl 4]. Inorganic chemistrycommunications, 6, 1129-1132.
7. P. P. Kulkarni, S. Padhye, E. Sinn, C. E. Anson, A. K. Powell. (2002) Comparative studies onCopper (I) complexes: synthesis, X-ray crystallography and electrochemical properties of[Cu I (dafone) n X] complexes (dafone= 4, 5-diaza-fluoren-9-one, X= Br, I, SCN). Inorganicachimica acta, 332, 167-175.
6. P. P. Kulkarni, S. Padhye, E. Sinn. (2001) Neutral metal complex in an ionic pocket: synthesis,physicochemical properties and X-ray structure of a copper (II) complex containing neutralas well as cationic dafone ligands and dafonium perchlorate. Inorganicachimica acta, 321, 193-199.
5. S. Padhye, P. P. Kulkarni, C. E. Anson, A. K. Powell, E. Sinn. (2001) Crystal structures of newpolymeric (4, 5-diaza-fluoren-9-one) Cu (I) complexes. POLYMER PREPRINTS-AMERICA,43, 380-381.
4. M. Gupta, R. K. Kale, P. P. Kulkarni, S. B. Padhye. (1999) Antioxidant and Prooxidant Effects ofMetal Dafonates on the NADPH-Dependent Lipid Peroxidation in the Murine HepaticMicrosomal System. Metal Based Drugs, 6, 169-176.
3. P. P. Kulkarni, S. Padhye, E. Sinn. (1998) The first well characterized Fe (phen) Cl3complex:structure of aquo mono(1,10-phenanthroline) iron (III) trichloride: [Fe (phen) Cl3 (H2O)]. Polyhedron, 17, 2623-2626.
2. A. Kumbhar, P. P. Kulkarni, S. Padhye. (1996) Cytotoxic properties of metal-quinone complexes:Role of redox cycling in the toxicities of iron (II) and iron (III) complexes of 2-and 5-hydroxy-1, 4-naphthoquinones to isolated rat hepatocytes. Journal of Chemical Sciences, 108, 315-315.
1. A. Kumbhar, S. Damle, P. P. Kulkarni, D. Srinivas, V. Chatur. (1996) Synthesis, characterizationand nuclease activity of Copper (II), nickel (II) and iron (II) complexes of 2-acetylpyridineoxime. Indian journal of chemistry. Sect. A: Inorganic, physical, theoretical & analytical, 35,533-535.